
What is gas chromatography (GC)?
Gas chromatography (GC) is a proven analytical method that can be used to identify and quantify individual components of a mixture of substances. It is primarily used to analyze gaseous and volatile substances and is one of the most important separation methods in modern analytics.
Unlike liquid-based methods, gas chromatography separates substances in a separation column through which a carrier gas flows. The method enables fast, precise, and cost-efficient analysis of even the smallest quantities of a mixture.
How does gas chromatography work?
In gas chromatography, a sample is introduced into the injector of a gas chromatograph and vaporized there. The resulting gas is transported through a so-called separation column with the aid of a carrier gas (e.g., helium or hydrogen). Within this column, the substances in the sample interact to varying degrees with the stationary phase, which adheres to the column wall.
These interactions cause the components of the sample to move through the column at different speeds. As a result, they leave the column at different times, known as retention times, and are detected one after the other by a detector.
The results are output as a chromatogram: a graphical representation with peaks, each of which represents a substance in the mixture.
A gas chromatograph therefore ensures that a sample can be analyzed automatically, in a controlled manner, and with high precision for its components, while maintaining defined temperature, pressure, and flow rate conditions.

Advantages of gas chromatography
Gas chromatography offers a number of advantages over other analytical methods. It is particularly suitable when volatile or easily evaporated compounds in a mixture need to be accurately identified and quantified.
What sets gas chromatography apart:
- High separation efficiency: Even very similar molecules can be reliably separated
- Fast analysis: Short run times enable a high number of samples per day
- High sensitivity: Detection of even the lowest concentrations of substances
- Wide range of applications: From food testing to environmental analysis
- Can be coupled with MS: Combination with mass spectrometers (GC-MS) allows even more precise results
- Cost-effective: Low operating costs and fast throughput
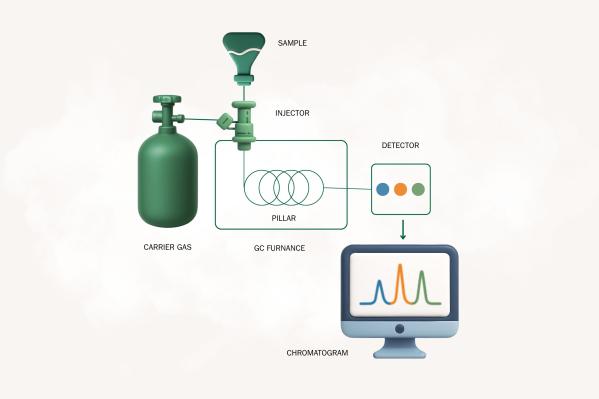
Structure of a gas chromatograph
Injector: This is where the sample is introduced and vaporized. Vaporization is necessary so that the sample can transition into the gaseous phase.
Carrier gas system: The carrier gas (usually helium, hydrogen, or nitrogen) transports the vaporized sample through the system. It must be very pure so as not to distort the results.
Separation column: The heart of the device. This is where the actual separation of the mixtures of substances takes place. A distinction is made between capillary columns and packed columns. In the stationary phase, which coats the inner wall of the column, interactions with the individual compounds occur.
Temperature control: An oven regulates the temperature inside the separation column, as the separation of many substances is highly temperature-dependent. This method is called temperature-programmed gas chromatography.
Detector: At the end of the column, the detector measures the concentration of the emerging analytes. The flame ionization detector is frequently used, but other types of detectors are also possible.
Data processing: The signals from the detector are displayed graphically in a chromatogram and stored for further evaluation.
The phases of gas chromatography
Gas Chromatography separates mixtures based on the different interactions of the components with two phases: the mobile phase (carrier gas) and the stationary phase (the coated separation column). The extent to which an analyte molecule interacts with the stationary phase determines its retention time—that is, how long it takes to pass through the column.
The Mobile Phase: the Carrier Gas
The mobile phase is an inert gas that transports the vaporized sample through the column.
Common carrier gases include:
- Helium (He): widely used, inert, highly reproducible
- Hydrogen (H₂): very fast separation, high efficiency, but flammable
- Nitrogen (N₂): inexpensive, but lower separation performance in long columns
The choice of carrier gas affects separation time, signal quality, and detector compatibility.
The Stationary Phase: the Heart of the Separation Column
The stationary phase is a thin film coated on the inner wall of the capillary column. It typically consists of polymeric materials whose chemical structure is tailored to the properties of the substances being separated.
Typical stationary phase materials include:
- Polydimethylsiloxane (PDMS): non-polar, standard phase for many non-polar compounds
- Poly(ethylene glycol) (PEG): highly polar, ideal for alcohols, aldehydes, organic acids
- Cyanopropylphenyl-Methylsiloxane: moderately polar, suitable for a wide range of applications
- Carbowax®: trade name for PEG-based material
- Waxes and modified polymers: for special matrix requirements
The separation performance depends heavily on the properties of the stationary phase, but also on the physical parameters of the column.
Influence of Length, Diameter, and Film Thickness
- Column length: Longer columns (up to 60 m) enable higher resolution but require longer analysis times.
- Inner diameter (ID): Smaller diameters (e.g., 0.25 mm) yield sharper peaks but demand precisely adjusted flow rates.
- Film thickness: Thicker films slow down separation (longer retention times) but increase sample capacity and improve peak shape for polar analytes.
For optimal separation, each mixture requires a carefully matched combination of carrier gas (mobile phase), stationary phase coating, and a separation column whose length, diameter, and film thickness are tailored to the specific analysis. The selection of these parameters is critical for resolution, signal quality, and ultimately, the reliability of the chromatogram.
Separation principle and retention time
The central goal of gas chromatography is the most precise possible separation of a mixture into its individual components. This is based on the different distribution of analytes between the mobile phase (carrier gas) and the stationary phase (column coating). This process is referred to as partition chromatography.
How does the separation work?
When a sample enters the separation column, its components interact with the stationary phase to varying degrees. Substances that interact more strongly with this phase (e.g., through van der Waals forces, polarity, or molecular size) move more slowly through the column than those with lower affinity.
These differing residence times cause the substances to exit the column at different points in time—known as their retention time.
Key factors influencing retention time:
- Chemical properties of the substance (polarity, boiling point, molecular mass)
- Column temperature (higher temperatures shorten retention time)
- Characteristics of the stationary phase
- Carrier gas flow rate
- Column geometry (length, diameter, film thickness)
The more similar two compounds are, the greater the challenge of separating them over time. In such cases, highly selective stationary phases and precise temperature programs play a crucial role.
The retention time is the most important parameter for identifying a substance in a chromatogram. High-quality separation is only possible when the physicochemical properties of the analytes, the characteristics of the stationary phase, and the operating conditions of the instrument are carefully aligned.
Detectors in gas chromatography
A detector is the component in a gas chromatograph that measures when and in what quantity an analyte molecule exits the separation column. Selecting the right type of detector is crucial for the sensitivity, selectivity, and applicability of an analysis.
How do detectors work?
A detector registers the physical or chemical properties of the substances leaving the column—such as electrical conductivity, thermal conductivity, or ionization behavior—and converts these into an electrical signal. This signal is displayed as a peak in the chromatogram.
The height of the peak directly correlates with the concentration of the analyte, while the retention time corresponds to its identity.
Common detector types in gas chromatography
1. Flame Ionization Detector (FID):
- The most commonly used detector in GC
- Burns organic compounds in a hydrogen flame
- Measures electrical currents generated by ionization
- Advantages: High sensitivity for nearly all organic substances, robust, wide linear dynamic range
2. Thermal Conductivity Detector (TCD):
- Measures changes in the thermal conductivity of the carrier gas when analytes exit the column
- Universal detector: can detect nearly all substances
- Less sensitive than FID, but non-destructive
3. Electron Capture Detector (ECD):
- Particularly sensitive to halogenated compounds (e.g., pesticides, PCBs)
- Utilizes the effect that certain molecules capture free electrons
- Highly selective, commonly used in environmental analysis
4. Mass Spectrometer (MS):
- Frequently used in combination with GC (GC-MS)
- Identifies molecules based on molecular mass and fragmentation patterns
- Enables structure-related information and compound identification
5. Other Detectors:
- Nitrogen-Phosphorus Detector (NPD): specific for nitrogen- and phosphorus-containing compounds
- Flame Photometric Detector (FPD): for sulfur and phosphorus analysis
- Photoionization Detector (PID): for aromatic hydrocarbons
The choice of detector depends on the substances being analyzed, the required detection limits, and the sample matrix. For many routine analyses, the FID is the first choice. For more specific applications, selective or structure-elucidating detectors such as the ECD or mass spectrometer are preferred.
Understanding chromatograms and peaks
The chromatogram is the central result of a gas chromatographic analysis. It is a graphical diagram that shows the detector’s signal over time. Each peak corresponds to a single analyte that exits the separation column at a specific time (retention time) and is detected.
Structure of a Chromatogram
- X-axis: Time (usually in minutes), indicating the retention time of each analyte
- Y-axis: Signal intensity, representing the concentration of the substance
- Peaks: Each peak represents a detected compound. The area under the peak is proportional to the amount of substance present.
What does a peak tell us?
- Position of the peak: Determines the identity of the substance via its retention time
- Height or area of the peak: Indicates the concentration or amount of the substance
- Shape of the peak: Ideally symmetrical. Asymmetrical peaks may point to overloading, matrix effects, or technical issues
Peak shapes and their meaning
- Narrow, tall peak: Good separation, high sensitivity
- Broad peak: Possibly insufficient separation or too low flow rate
- Double or overlapping peaks: Indicate incomplete separation of components
- Tailing or fronting: Distorted peaks, often caused by interactions with the injector or column
A correctly interpreted chromatogram provides both qualitative and quantitative information about a mixture. For accurate results, not only the separation itself but also the clear representation of peaks is critical. Signal shape, retention time, and peak area are the key factors for identifying and quantifying individual compounds.

Areas of application in analytics
Gas Chromatography (GC) is a proven method for analyzing volatile and semi-volatile compounds, with a wide range of applications in the food, environmental, cosmetics, and pharmaceutical industries.
Qualitative Analyses: Which substances are present?
In qualitative analysis, gas chromatography identifies individual compounds based on their retention time in the chromatogram. By comparing with reference standards, it is possible to determine which substances are present in a sample.
Typical applications:
- Residue analysis of pesticides in fruits and vegetables
e.g., ensuring compliance with legal maximum residue levels in food testing - Detection of solvent residues in cosmetics
e.g., testing of essential oils or perfume components - Determination of aroma compounds in beverages and spirits
e.g., authenticity control in wine and beverage testing - Screening for environmental contaminants in soil or water samples
e.g., analysis of PAHs, VOCs, or pesticides in environmental monitoring
The qualitative GC analysis provides a precise chemical profile for product safety, authenticity testing, or the detection of unwanted contaminants.
Quantitative Analyses: How much is present?
For quantitative analysis, GC measures the area under the peaks, which is proportional to the concentration of the respective substance.
Typical applications:
- Determination of mineral oil residues (MOSH/MOAH) in foods
e.g., in chocolate, baked goods, or from packaging influences - Measurement of residual solvents in dietary supplements
e.g., after manufacturing processes involving ethanol, hexane, etc. - Quantification of residues from cleaning agents in cosmetics
e.g., detection of isopropanol, acetone, or limonene - Determination of residual alcohol in “alcohol-free” beverages
ensuring compliance with the 0.5% vol. limit
GC-MS coupling: Why and when?
In classical gas chromatography (GC), compounds are identified by their characteristic retention time. However, this information alone is not always sufficient to determine a substance unambiguously—for example, in cases of overlapping peaks, similar molecular structures, or complex matrices. In such situations, GC is combined with a mass spectrometer, a technique known as GC-MS.
How does GC-MS work?
The GC-MS coupling combines two powerful methods:
- Gas chromatography separates the components of the sample over time.
- Mass spectrometry then analyzes each molecule individually by ionizing it and breaking it down into characteristic fragmentation patterns.
These patterns, known as mass spectra, are unique and serve as molecular "fingerprints," allowing substances to be identified with high precision.
Advantages of GC-MS coupling
- Unambiguous identification even for very similar substances
- Highest sensitivity for trace analysis in the low ppb or ppt range
- Structural elucidation of unknown compounds through characteristic fragmentation
- Reliable results even with complex sample matrices
GC-MS is particularly valuable when no clear reference values for retention times exist, or when unexpected compounds are detected. It is also widely used for legally regulated substance groups, such as pesticides or environmental contaminants, to provide definitive evidence.
Gas chromatography at Tentamus: precise analysis for your products
Gas chromatography is a highly advanced technique that, in practice, requires precise adjustment of numerous factors—from selecting the right separation columns and detectors to interpreting complex chromatograms. Our laboratories work with state-of-the-art instrumentation and validated methods, tailored to your specific requirements.
Rely on our years of experience, personal consultation, and the quality of a laboratory network accredited according to DIN EN ISO/IEC 17025.
Our expert teams support you with:
- Selection of suitable GC methods for your sample
- Qualitative and quantitative analyses
- Residue analysis, identity verification, and contamination control
- Validation and documentation for regulatory compliance
Would you like to learn more about our gas chromatographic services or request an analysis?
FAQ: Frequently asked questions about gas chromatography
1.How long does gas chromatography take?
Depending on the method, a GC analysis takes from a few minutes up to about an hour. The exact duration depends on the type of sample, column length, and temperature program. Including sample preparation, evaluation, etc., results are usually available within a few working days.
2.How much does a gas chromatography analysis cost?
The costs vary depending on the depth of analysis, sample matrix, and detection method.
3.What is the difference between GC and HPLC?
GC works with vaporized samples and a carrier gas, while HPLC works with liquid samples and a liquid phase. GC is suitable for volatile substances, while HPLC is used for non-volatile and thermolabile compounds.
Read more about the differences between GC and HPLC here.
4.What is a chromatogram?
A chromatogram is the graphical representation of detector signals over time. Each peak corresponds to a specific substance in the mixture.
5.What does retention time mean?
Retention time is the period a substance requires to travel from injection to elution through the separation column. It serves to identify the substance.
6.What is the difference between an internal and an external standard?
An internal standard is added directly to the sample and serves as a reference in the same run. An external standard is analyzed separately and serves for comparison with the sample measurement.
7.What is headspace GC?
Headspace GC analyzes only the gaseous components above a sample, e.g., volatile aroma compounds or solvents. It is particularly gentle on the matrix.
8.Which substances cannot be analyzed with gas chromatography?
Non-volatile, thermally unstable, or very large molecules such as proteins or polymers usually cannot be analyzed using GC.
9.Which factors influence separation performance in GC?
The separation performance in gas chromatography is influenced by several physical and chemical factors. A central role is played by the choice of separation column, particularly its length, internal diameter, and the thickness of the stationary phase. The temperature program in the column oven—whether isothermal or temperature-programmed—also directly affects the separation quality.
Other influencing factors include the carrier gas (type and flow rate) as well as the chemical properties of the substances to be separated, such as polarity, boiling point, and molecular size. Only when all these parameters are carefully aligned can complex mixtures be separated with high resolution.
10.Who developed gas chromatography?
Modern gas chromatography was introduced in 1952 by Anthony T. James and Archer J.P. Martin, who later received the Nobel Prize for this work.